The Heisenberg Uncertainty Principle is a key part of quantum mechanics. It changes how we see reality. Introduced by German physicist Werner Heisenberg in 1927, it shows that some things can’t be known at the same time.
For example, you can’t know where something is and how fast it’s moving at the same time. This is different from classical physics, where we can know everything for sure. This principle shows us the limits of what we can know.
So, why is this important? Without it, atoms would collapse, stars wouldn’t shine, and technologies like semiconductors and MRI scans couldn’t exist.
The uncertainty principle is not a problem with measuring things. It’s a basic part of how the world works.
It helps explain why things stay stable and why quantum mechanics uses probability. It also shows how modern technology works by balancing what we know and what we don’t.
Key Takeaways
- Reality has built-in limits: Certain pairs of properties (like position and momentum) can’t be precisely measured at the same time.
- Quantum stability: This principle stops atoms from collapsing and enables stars to produce energy.
- Not a measurement flaw: The uncertainty comes from nature’s design, not human limitations.
- Heisenberg Uncertainty Principle: From solar energy to advanced technology, this idea shapes our world in unseen ways.
- New perspective: Accepting uncertainty helps us understand larger systems, from particles to galaxies.
Overview of Quantum Concepts and the Heisenberg Uncertainty Principle
Picture a world where certainty dissolves into probability. At subatomic scales, according to quantum theory, particles defy common sense—acting like spread-out waves one moment and pinpoint dots the next.
Quantum mechanics reveals how two opposing truths can exist together. This is similar to the Duality Mental Model.
This quantum realm operates on different rules, where the uncertainty principle makes precise knowledge impossible by design. The Heisenberg uncertainty principle illustrates how position and momentum cannot be simultaneously known with accuracy.
Breaking Classical Expectations
In our daily lives, objects follow predictable paths. A thrown ball arcs through set coordinates. Quantum systems work differently.
This clash between certainty and probability is like the Recency Mental Model. It shows how the Recency bias often overshadows larger probability trends.
Electrons don’t circle nuclei like planets—they form probability clouds showing where they might be. This isn’t measurement error; it’s a fact of the uncertainty principle and how nature maintains atomic stability through precise measurements of particles.
| Classical World | Quantum World | Real-World Impact |
|---|---|---|
| Definite positions | Probability distributions | Stable atoms |
| Predictable motion | Uncertain momentum | Functional semiconductors |
| Separate waves/particles | Dual behavior | MRI technology |
Heisenberg Uncertainty Principle: Duality Drives Discovery
Light’s split personality—acting as waves and particles—paved the way for quantum theory. Physicists like Bohr and Schrödinger realized energy flows in chunks (quanta), not smooth streams.
This duality creates inherent limits: observe a particle’s position, and its speed blurs. Measure its path, and location becomes fuzzy.
Wave-particle duality is similar to the Trade-Off Mental Model. It shows that gaining clarity in one area can mean losing it in another.
Modern tech relies on this quantum dance. Semiconductor chips use electron clouds to store data. MRI machines harness spinning particles’ uncertain states. What seems chaotic enables precision—proving that embracing uncertainty fuels progress.
Exploring the Heisenberg Uncertainty Principle in Detail

Imagine trying to catch fireflies in the dark. Shine a bright light, and you’ll see where they are—but the light’s energy from the photon scatters them instantly. This mirrors quantum measurement: knowing where something is often means losing track of how fast it’s moving.
The trade-off isn’t accidental—it’s nature’s design. It echoes the Probability Mental Model, where outcomes can only be described in terms of likelihood, not certainty.
Balancing Position and Momentum
Measure a particle’s position precisely, and its momentum becomes fuzzy. Why? Tools like photons carry energy. When they hit particles, they transfer momentum—like tapping a rolling marble.
The act of seeing changes what’s seen. This isn’t human error. It’s a fundamental rule written into reality.
Natural Limits: Why Tools Can’t Break the Rule
Better tools won’t bypass these limits. Atoms stay stable because particles can’t be pinned down. If electrons stopped moving, they’d spiral into nuclei. Uncertainty acts as a safety net—keeping matter intact.
| Measurement Focus | What We Gain | What We Lose |
|---|---|---|
| Exact Position | Pinpoint location | Speed data |
| Precise Momentum | Velocity details | Location clarity |
| Simultaneous Attempt | Partial data | Complete picture |
Everyday objects hide this dance. But in quantum realms, it’s unavoidable. Even empty space hums with temporary particles—flashes of “maybe” that shape our universe.
Mathematical Foundations of the Heisenberg Uncertainty Principle
Numbers don’t lie—but in quantum physics, they play by different rules. At its core, the relationship between position and momentum follows a strict equation: Δx·Δp ≥ h/4π.
This isn’t just math. It’s nature’s way of saying, “Choose your focus—you can’t have both.”
Understanding Δx·Δp ≥ h/4π (H3)
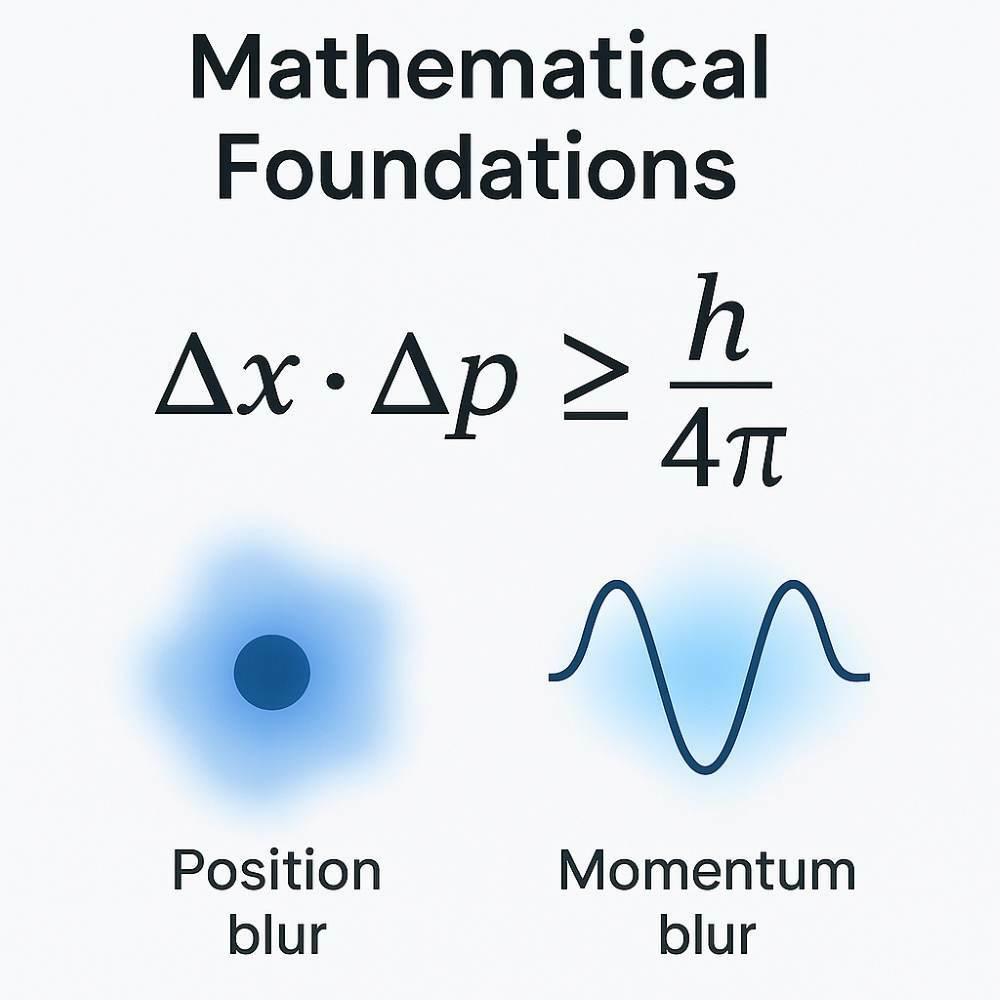
Let’s break it down. Δx measures how fuzzy a particle’s location is. Δp tracks the blur in its momentum. Multiply them, and the result must always meet or exceed h/4π—Planck’s constant (6.626×10⁻³⁴ Js) divided by four pi.
This tiny number acts like a cosmic speed limit for precision.
Why two versions of the formula? Physicists often use ℏ/2 instead, where ℏ (h-bar) equals h/2π. It’s the same rule, just simplified. Think of it as switching between miles and kilometers—the journey stays the same.
Derivation Using de Broglie Wavelength
Louis de Broglie revealed particles have wave-like traits. His equation λ = h/p connects wavelength (λ) to momentum (p).
Sharply defined momentum means a clear wave pattern—but that spreads out position uncertainty. Tighten the location, and the wave becomes chaotic, scrambling momentum data.
Try this: Calculate an electron’s speed within 1% accuracy. The math shows its position blurs by an atom’s width. These aren’t errors—they’re baked into reality’s recipe.
Even energy and time follow similar rules, proving quantum limits shape everything from lab experiments to starlight.
Historical Background and Experimental Proofs

Scientific revolutions often begin with a single question. In 1927, a young German physicist working in Copenhagen dared to ask: What can we truly know about particles?
His answer reshaped physics forever, leading to what we now understand as the uncertainty principle.
This principle says that the more precisely we know a particle’s position, the less precisely we can know its momentum, as described by the Planck constant.
Werner Heisenberg’s 1927 Paper
Werner Heisenberg’s work was a game-changer. He introduced matrix mechanics and the Uncertainty Principle. This breakthrough came through his letters to Wolfgang Pauli.
Their exchanges refined ideas later published as “On the Perceptual Content of Quantum Theoretical Kinematics and Mechanics”. This paper introduced a radical concept—measurement alters reality at quantum scales.
Heisenberg’s thought experiment used gamma-ray photons to imagine observing electrons. Like using a flashlight to find marbles in the dark, high-energy light reveals position but scatters momentum. Heisenberg’s theoretical microscope showed why perfect precision breaks down—not from human limits, but nature’s rules.
The 1988 Neutron Experiment
Six decades later, scientists tested these ideas with neutrons. At France’s Institut Laue-Langevin, researchers measured particle position and momentum simultaneously. The results matched Heisenberg’s predictions—sharpening one measurement blurred the other.
This experiment proved quantum uncertainty isn’t theoretical. It’s woven into matter itself. Neutrons acted as both particles and waves, their dual nature enforcing the 1927 paper’s claims.
Today, this balance enables technologies from solar panels to medical imaging.
Applications of the Heisenberg Uncertainty Principle in Modern Technology
Your smartphone holds secrets written in quantum code. The same rules that baffle physicists power devices we use daily. From instant photo storage to life-saving medical scans, quantum behavior shapes our tools in unexpected ways.
Quantum Tunneling and Flash Memory
Flash drives work because electrons break classical rules. When you save a file, particles tunnel through solid barriers—like ghosts walking through walls. This quantum behavior lets devices store data without power. Without this effect, your photos would vanish when the battery dies.
Semiconductors and MRI Systems
Computer chips thrive on controlled uncertainty. Electrons flow through semiconductors as probability clouds, not defined paths. MRI machines use similar principles—tracking spinning nuclei whose positions blur while their motion stays measurable.
This balance enables detailed body scans without invasive surgery.
Today, over 2 billion MRI scans have been done globally. They all rely on Heisenberg’s groundbreaking discovery.
| Technology | Quantum Process | Real-World Use |
|---|---|---|
| Flash Memory | Tunneling through barriers | Data storage |
| Semiconductors | Electron cloud behavior | Smartphones |
| MRI Machines | Nuclear spin states | Medical imaging |
Quantum computers take this further. Their qubits exist in multiple states at once, solving problems faster than classical machines. Even sunlight relies on quantum uncertainty—protons fuse in the sun’s core through tunneling, creating energy that reaches Earth.
Conclusion: Why Uncertainty Creates Stability
The universe keeps its deepest truths just out of reach. Reality operates on a cosmic rulebook where precision has limits. This is how life becomes possible. The Heisenberg Uncertainty Principle reminds us that uncertainty creates stability.
This principle shapes both the smallest atoms and the brightest stars. It shows how limits can drive innovation. Without it, smartphones and stars wouldn’t exist.
From smartphones to probability models guiding decisions, this principle is key. If particles followed classical rules, stars would flicker out, and atoms would collapse.
Next time you charge your phone or gaze at the night sky, remember. Uncertainty isn’t a barrier. It’s the quiet architect of everything we know.
For more insights, explore the Trade-Off Mental Model. It shows how gaining clarity in one area often means letting go in another. Like quantum physics, it reveals the balance between them.


